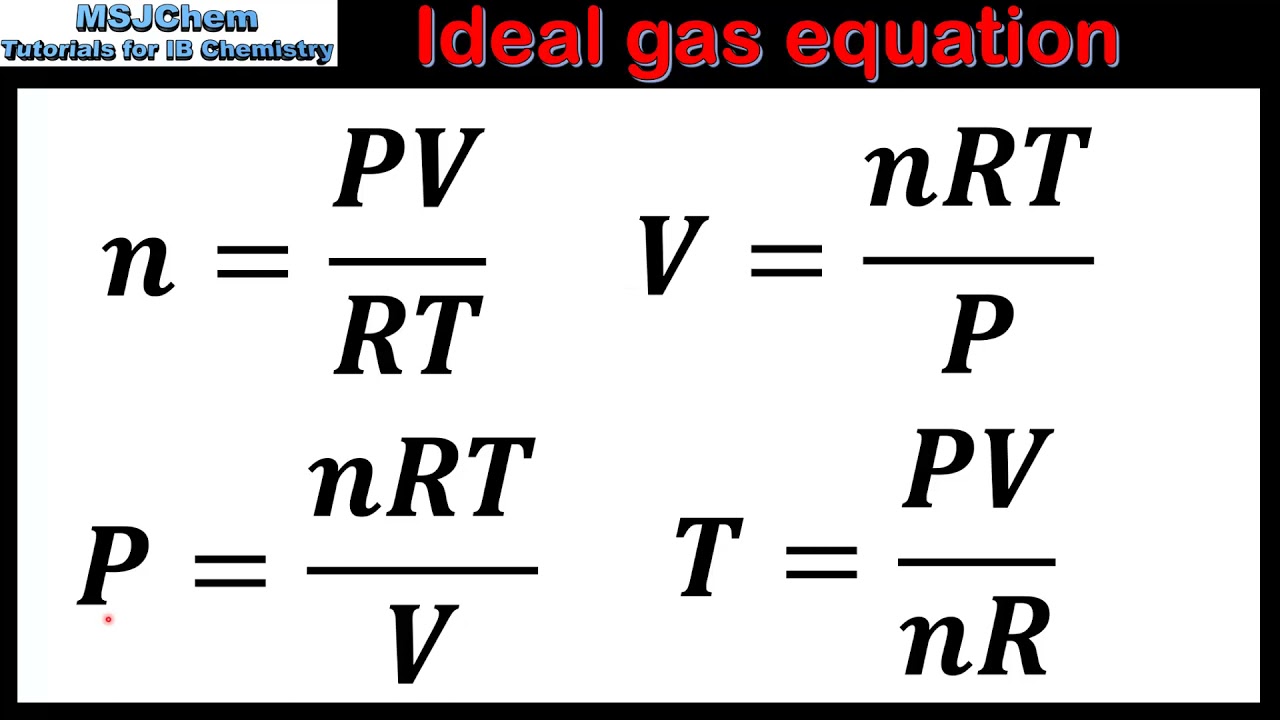
How To Find Pressure Using Ideal Gas Law . use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given. To calculate the gas pressure using the ideal gas law, follow. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Use the ideal gas law to calculate pressure change,. The ideal gas law states that pv = nkt, where p is the absolute pressure of a gas, v is the volume it occupies, n is the number of atoms and. Use the ideal gas law to calculate pressure change,. state and explain the ideal gas law using boltzmann's constant; how do you calculate pressure using ideal gas law? the ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and. state the ideal gas law in terms of molecules and in terms of moles.
from worksheetfullsmokers.z22.web.core.windows.net
To calculate the gas pressure using the ideal gas law, follow. The ideal gas law states that pv = nkt, where p is the absolute pressure of a gas, v is the volume it occupies, n is the number of atoms and. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. state the ideal gas law in terms of molecules and in terms of moles. Use the ideal gas law to calculate pressure change,. how do you calculate pressure using ideal gas law? the ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and. state and explain the ideal gas law using boltzmann's constant; Use the ideal gas law to calculate pressure change,.
How To Calculate Gas Law
How To Find Pressure Using Ideal Gas Law Use the ideal gas law to calculate pressure change,. state and explain the ideal gas law using boltzmann's constant; use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given. Use the ideal gas law to calculate pressure change,. the ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. how do you calculate pressure using ideal gas law? Use the ideal gas law to calculate pressure change,. state the ideal gas law in terms of molecules and in terms of moles. The ideal gas law states that pv = nkt, where p is the absolute pressure of a gas, v is the volume it occupies, n is the number of atoms and. To calculate the gas pressure using the ideal gas law, follow.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download How To Find Pressure Using Ideal Gas Law state the ideal gas law in terms of molecules and in terms of moles. Use the ideal gas law to calculate pressure change,. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given. Use the ideal gas law to calculate pressure change,. The ideal gas. How To Find Pressure Using Ideal Gas Law.
From byjus.com
Ideal Gas Law Equation Compressibility Of Natural Gas Chemistry How To Find Pressure Using Ideal Gas Law To calculate the gas pressure using the ideal gas law, follow. how do you calculate pressure using ideal gas law? the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. state the ideal gas law in terms of molecules and in terms of moles. Use the ideal. How To Find Pressure Using Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 How To Find Pressure Using Ideal Gas Law the ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and. To calculate the gas pressure using the ideal gas law, follow. The ideal gas law states that pv = nkt, where p is the absolute pressure of a gas, v is the volume it occupies, n is the number of atoms and.. How To Find Pressure Using Ideal Gas Law.
From www.youtube.com
PVM mRT Ideal Gas law 2 YouTube How To Find Pressure Using Ideal Gas Law The ideal gas law states that pv = nkt, where p is the absolute pressure of a gas, v is the volume it occupies, n is the number of atoms and. Use the ideal gas law to calculate pressure change,. the ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and. state. How To Find Pressure Using Ideal Gas Law.
From learningravnihym.z21.web.core.windows.net
Combined Gas Law Explanation How To Find Pressure Using Ideal Gas Law The ideal gas law states that pv = nkt, where p is the absolute pressure of a gas, v is the volume it occupies, n is the number of atoms and. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given. Use the ideal gas law. How To Find Pressure Using Ideal Gas Law.
From www.youtube.com
Ideal Gas Law Total Pressure of Two Flasks YouTube How To Find Pressure Using Ideal Gas Law Use the ideal gas law to calculate pressure change,. To calculate the gas pressure using the ideal gas law, follow. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. the ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and. Use. How To Find Pressure Using Ideal Gas Law.
From www.youtube.com
Ideal gas, How many moles of gas and what is the pressure YouTube How To Find Pressure Using Ideal Gas Law the ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and. To calculate the gas pressure using the ideal gas law, follow. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given. how do you calculate pressure using. How To Find Pressure Using Ideal Gas Law.
From www.youtube.com
Ideal Gas Law PV=nRT Finding Pressure YouTube How To Find Pressure Using Ideal Gas Law To calculate the gas pressure using the ideal gas law, follow. state and explain the ideal gas law using boltzmann's constant; Use the ideal gas law to calculate pressure change,. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given. the ideal gas law. How To Find Pressure Using Ideal Gas Law.
From www.slideshare.net
Ideal gas law practice mccpot How To Find Pressure Using Ideal Gas Law Use the ideal gas law to calculate pressure change,. Use the ideal gas law to calculate pressure change,. The ideal gas law states that pv = nkt, where p is the absolute pressure of a gas, v is the volume it occupies, n is the number of atoms and. the ideal gas law is derived from empirical relationships among. How To Find Pressure Using Ideal Gas Law.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID How To Find Pressure Using Ideal Gas Law the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. the ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles. How To Find Pressure Using Ideal Gas Law.
From www.youtube.com
11.6 The Combined Gas Law Pressure, Volume, & Temperature YouTube How To Find Pressure Using Ideal Gas Law The ideal gas law states that pv = nkt, where p is the absolute pressure of a gas, v is the volume it occupies, n is the number of atoms and. Use the ideal gas law to calculate pressure change,. how do you calculate pressure using ideal gas law? Use the ideal gas law to calculate pressure change,. . How To Find Pressure Using Ideal Gas Law.
From www.alamy.com
Ideal gas law formula. Pressure, volume, amount of substance , ideal How To Find Pressure Using Ideal Gas Law use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given. how do you calculate pressure using ideal gas law? To calculate the gas pressure using the ideal gas law, follow. the ideal gas law is derived from empirical relationships among the pressure, the volume,. How To Find Pressure Using Ideal Gas Law.
From www.youtube.com
Ideal Gas Law Practice Problems YouTube How To Find Pressure Using Ideal Gas Law state and explain the ideal gas law using boltzmann's constant; the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Use the ideal gas law to calculate pressure change,. The ideal gas law states that pv = nkt, where p is the absolute pressure of a gas, v. How To Find Pressure Using Ideal Gas Law.
From www.grc.nasa.gov
Equation of State How To Find Pressure Using Ideal Gas Law state the ideal gas law in terms of molecules and in terms of moles. To calculate the gas pressure using the ideal gas law, follow. how do you calculate pressure using ideal gas law? the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. state and. How To Find Pressure Using Ideal Gas Law.
From www.youtube.com
IDEAL GAS LAW PRACTICE PROBLEMS How to Solve Ideal Gas Law Problems How To Find Pressure Using Ideal Gas Law To calculate the gas pressure using the ideal gas law, follow. Use the ideal gas law to calculate pressure change,. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. state and explain the ideal gas law using boltzmann's constant; the ideal gas law is a combination. How To Find Pressure Using Ideal Gas Law.
From www.youtube.com
11.8 The Ideal Gas Law Pressure, Volume, Temperature, & Moles YouTube How To Find Pressure Using Ideal Gas Law The ideal gas law states that pv = nkt, where p is the absolute pressure of a gas, v is the volume it occupies, n is the number of atoms and. the ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and. use the ideal gas law to calculate pressure change, temperature. How To Find Pressure Using Ideal Gas Law.
From www.chegg.com
Solved Find the pressure using the ideal gas law and How To Find Pressure Using Ideal Gas Law The ideal gas law states that pv = nkt, where p is the absolute pressure of a gas, v is the volume it occupies, n is the number of atoms and. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. state and explain the ideal gas law. How To Find Pressure Using Ideal Gas Law.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID How To Find Pressure Using Ideal Gas Law state and explain the ideal gas law using boltzmann's constant; the ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given. Use the ideal gas law to calculate. How To Find Pressure Using Ideal Gas Law.